Repurposed Drug Helps Cells Clear Defective Proteins, Reveals Route to New Therapies
February 29, 2024
A decades-old drug for rheumatoid arthritis (RA) could point the way to a new class of treatments for diseases like cancer and Alzheimer’s disease.
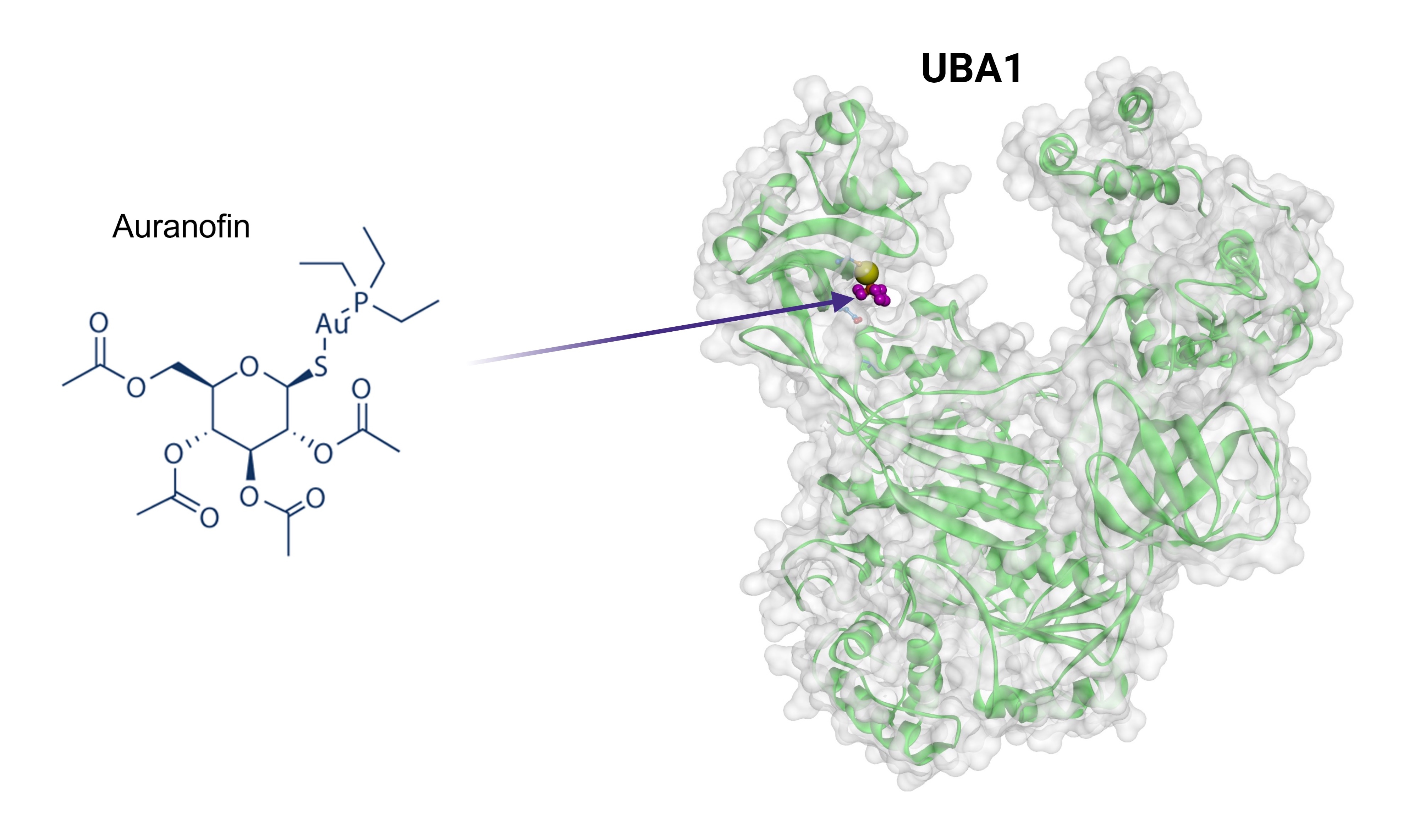
A research team from NCATS, the National Institute of Diabetes and Digestive and Kidney Diseases, and the University of Maryland, Baltimore (UMB) found that auranofin, a gold-based compound approved in 1985 to treat RA, can increase cells’ ability to clear out defective proteins, a process called ubiquitination. Their findings appear in the journal Nature Communications.
When ubiquitination stops working, unwanted proteins can pile up, harming a cell’s normal functions. This pile-up can fuel aging and a range of both common and rare diseases, including cancer; disorders that damage or destroy parts of the nervous system, such as Parkinson’s disease; and rare conditions, such as VEXAS syndrome.
“All of these diseases have common problems with protein degradation,” explained NCATS scientist Dingyin Tao, Ph.D., one of the study’s two corresponding authors. “Our findings have the potential to enhance the protein degradation pathway and offer a new therapeutic approach for these diseases.”
A Golden Link to UBA1
The research team learned of auranofin’s potential during its search for drugs that changed how cells remove unwanted proteins. They found that cells treated with auranofin showed better protein removal. A closer look showed that auranofin acted on an enzyme called UBA1.
“UBA1 sits at the very top of the ubiquitination cascade,” explained NCATS scientist Mark J. Henderson, Ph.D., a study coauthor. “Using auranofin, you can increase the activity of that entire cascade.”
UBA1 triggers the first step in a three-part chain of events that activates the protein ubiquitin and starts ubiquitination. UBA1 grabs ubiquitin and connects to one of a group of enzymes known as E2 enzymes. UBA1 then passes ubiquitin to an E2 enzyme, which leads to the second step in the cascade.
The scientists found that auranofin acts like molecular glue to hold UBA1 and the E2 enzyme together. That stronger bond could increase the chances that the cascade’s first step will lead to step two, then move to step three and beyond.
Tests proved that the drug did, in fact, lead to that desired downstream effect. The researchers studied the degradation of several key proteins in cells treated with the drug. After treatment, all of those proteins were removed faster.
UBA1 Enhancer Could Lead to New Therapies
Not only did auranofin greatly strengthen UBA1, but it did so at concentrations up to 73 times lower than its approved dose for RA. That lower-dose success could reduce the risk of auranofin’s known side effects if it were repurposed as a UBA1 therapy.
Auranofin’s promise could make it a therapeutic candidate to treat rare conditions caused by faulty or inactive UBA1, including inflammatory VEXAS syndrome, spinal muscular atrophy, and some types of lung cancer in people who have never smoked. The results also could offer a starting point for designing new small molecules to strengthen UBA1 activity and improve cells’ protein-removal process.
“While UBA1 inhibitors have been reported for years, our discovery marks the first demonstration that UBA1 activity can be enhanced by a small-molecule drug,” said corresponding author Shengyun Fang, M.B.B.S., Ph.D., professor at UMB. “This discovery lays the groundwork for repurposing auranofin and developing novel and specific enhancers for UBA1 activity, addressing the imperative needs for treating numerous debilitating diseases."